Which Statement Best Describes an Oxidation Reduction Reaction
The reactants are two electrically neutral elements. The product however is ionic.

Chemistry Notes Teaching Science Chemistry Class
The following is an example of a combustion reaction.

. If there is formation of a precipitate the reaction is an oxidation-reduction reaction. Memorize flashcards and build a practice test to quiz yourself before your exam. Redox reactions involve formal electron transfer and a FORMAL change in oxidation number.
D Silver can displace copper from an aqueous solution of. Which best identifies why the rusting of an iron nail in the presence of water and oxygen is an oxidation-reduction reaction. Carbon dioxide is often produced as a product.
Consider this chemical reaction. A chemical reaction that involves oxygen. The following equations are half reactions and reduction potentials.
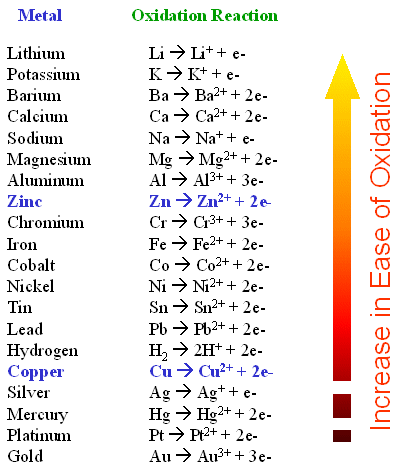
Cr3 aq 3e- - Crs has a reduction potential of -074 V. Third option is the correct one. Chemistry 19092021 2310 NetherisIsTheQueen.
It is composed of Mg 2 and Cl ions. Somehow the individual Mg atoms lose two. Recognize a reaction as an oxidation-reduction reaction.
1Which statement best describes an oxidation-reduction reaction1 point A. Which statement best describes the following reaction. A An oxidation reaction can proceed only in the company of a reduction reaction.
A chemical reaction in which electrons are transferred between reactants. C The precipitate of copper sulphide is red in colour. Mg s Cl 2 g MgCl 2.
Co2g H2g COg H2Ol What is being oxidized. A chemical reaction in which electrons are released from the system D. Image Zerovalent copper is OXIDIZED to C u 2 and A g is reduced to silver metal A 0 g.
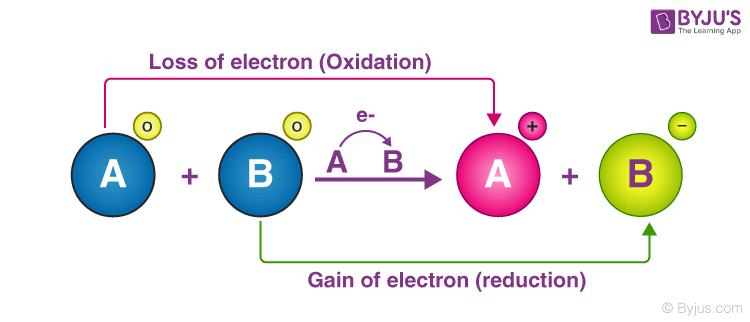
A chemical reaction in which electrons are released from the system. Recall that O has an oxidation number of -2 Electrons are transferred. So we write the individual reactions with the oxidation number of each constituent species superscripted.
C decomposition reaction. Which statement best compares the substances in these half reactions. Hydrogen Consider the following reaction.
But when an element is reduced it gains electrons. Start studying the Oxidation-Reduction Quiz flashcards containing study terms like Which describes the oxidizing agent in a chemical reaction Given the reaction below which is the oxidized substance. Which of the following is a simple definition of reduction.
Iron is oxidized to form rust. In this reaction both iron and copper are oxidized. When an element is oxidized it loses electrons.
Which statement best describes the oxidizing and reducing abilities of the reactants. Fe Cu2 Fe2 Cu a. Silver ion Ag is a stronger oxidizing agent than copper ion Cu2 and copper metal is a stronger reducing agent than silver.
This is the basis of redox reactions. The correct statement that describes a Redox reaction is D. Which answer best describes what is happening in the following redox reaction.
This is not an oxidation-reduction reaction. Mg Cl2 Mg2 2Cl Which best identifies why the rusting of an iron nail in the. 4Fe 3O2 mc023-1jpg 2Fe2O3.
Which best identifies why the rusting of an iron nail in the presence of water and oxygen is an oxidation-reduction reaction. A Redox reaction oxidation-reduction reaction involves the exchangetransfer. In this reaction iron is reduced and copper is oxidized.
However an oxidizing agent oxidizes something else and gets reduced therefore gaining electrons. They have the same number of electrons as protons. In this reaction both iron and copper are reduced.
A chemical reaction in which there are fewer products than reactants B. Up to 10 cash back A combustion reaction is characterized as an oxidation-reduction reaction because the oxidation number of the reactants changes and oxygen is used to burn a fuel molecule. The type of reaction that is shown is.
B Ionic reactions taking place in aqueous solution are generally fast. A reduction-oxidation or redox reaction is a type of chemical reaction in which reduction and oxidation occur at the same time. A chemical reaction in which electrons are transferred between reactants.
A chemical reaction in which there are fewer products than reactants. The reduced species receives electrons whereas the oxidised species loses them. A chemical reaction that involves oxygen C.
O O O If there is a change in the oxidation states of atorns from reactants to products the reaction is an oxidation- reduction reaction If there is formation of salt and water the reaction is an oxidation-reduction reaction. In this reaction iron is oxidized and copper is reduced. The chemical formula that shows the correct subscripts is D BeF₂.
An oxidation process does not need the presence of oxygen despite its name. 1Which statement best describes an oxidation-reduction reaction. Ag aq e- - Ags has a reduction potential of 080 V.
The gain of. ZnSs 2O2g ZnSO4s.

Oxidation Reduction An Overview Sciencedirect Topics
Is Cellular Respiration An Oxidation Or Reduction Reaction
No comments for "Which Statement Best Describes an Oxidation Reduction Reaction"
Post a Comment